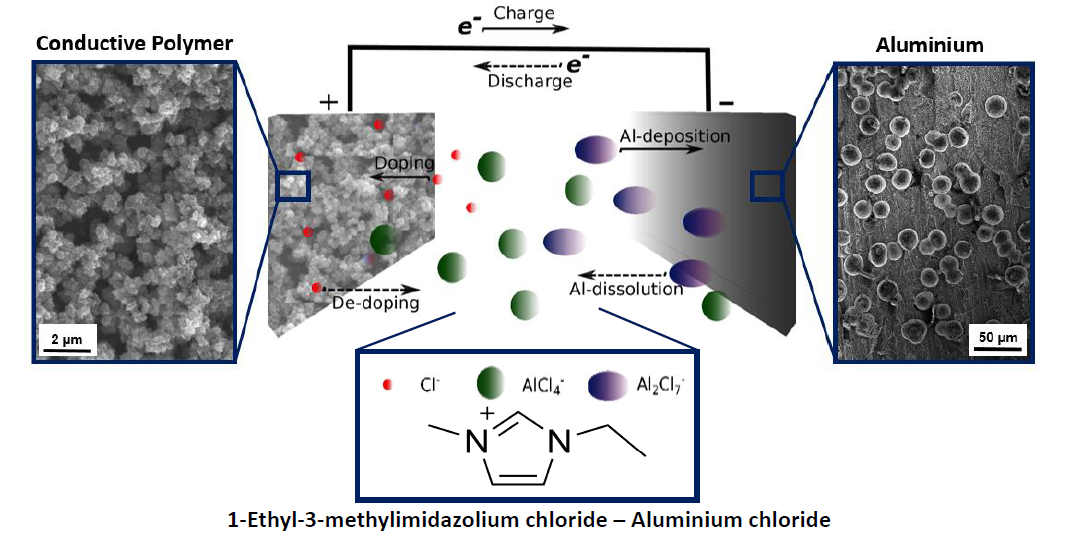
 Theresa Schoetz focused her PhD on a new concept of a non-aqueous rechargeable energy storage technology based on aluminium and a conductive polymer as the active electrode materials in a chloroaluminate ionic liquid. The principle is outlined on the left Figure.
Theresa Schoetz focused her PhD on a new concept of a non-aqueous rechargeable energy storage technology based on aluminium and a conductive polymer as the active electrode materials in a chloroaluminate ionic liquid. The principle is outlined on the left Figure.
The motivation of the project was the study of an energy storage technology which combines high specific energy and power together with sustainability and low costs, which are fundamental for an energy sustainable society, especially in terms of electro-mobility and consumer electronics products. This concept offers advantages over the current high-performance lithium-ion batteries which suffers sustainability issues such as recycling, limited resources, and thermal runaway.
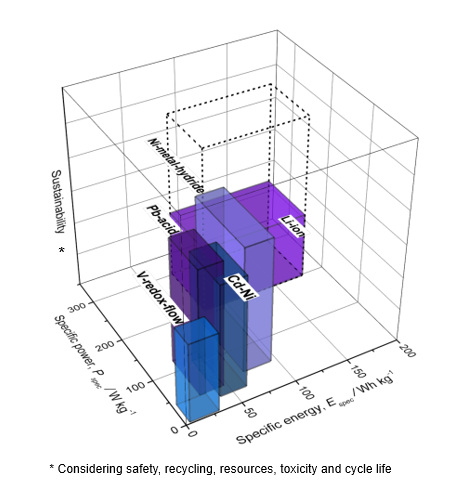
The novelty of the aluminium polymer battery combines the characteristics of a battery and a capacitor potentially offering the benefits of high specific energy and power of a hybrid battery-capacitor in one system. In addition, the materials stand out by their sustainability. See Figure below that compares several batteries with the ideal Sustainable High-Performance Batteries:
 |

Dr Theresa Schoetz |
Although aluminium is highly abundant, highly recyclable, cost-effective, lightweight and shows comparable specific capacities to lithium, aluminium based batteries in non-aqueous systems, have received less attention than lithium. In this project aluminium was combined with a charge storage material conductive polymer, which undergo a redox reaction like in a battery but at the same time can perform the characteristics of a capacitor by being doped/charged (de-doped/discharged) by anions contained in the battery electrolyte like non-toxic and highly stable ionic liquid. These so-called hybrid-battery-capacitor systems combine the faradaic and capacitive properties in a single electrode, providing the possibility to improve the cell performance significantly.
This polymer-aluminium battery was focussed on the conductive polymer electrode as it significantly determines the performance of the whole battery system. The studies comprised the synthesis of novel conductive polymer electrodes and their characterisation in ionic liquid electrolytes as well as the testing of the battery system and comparison with state-of-the-art rechargeable battery systems.
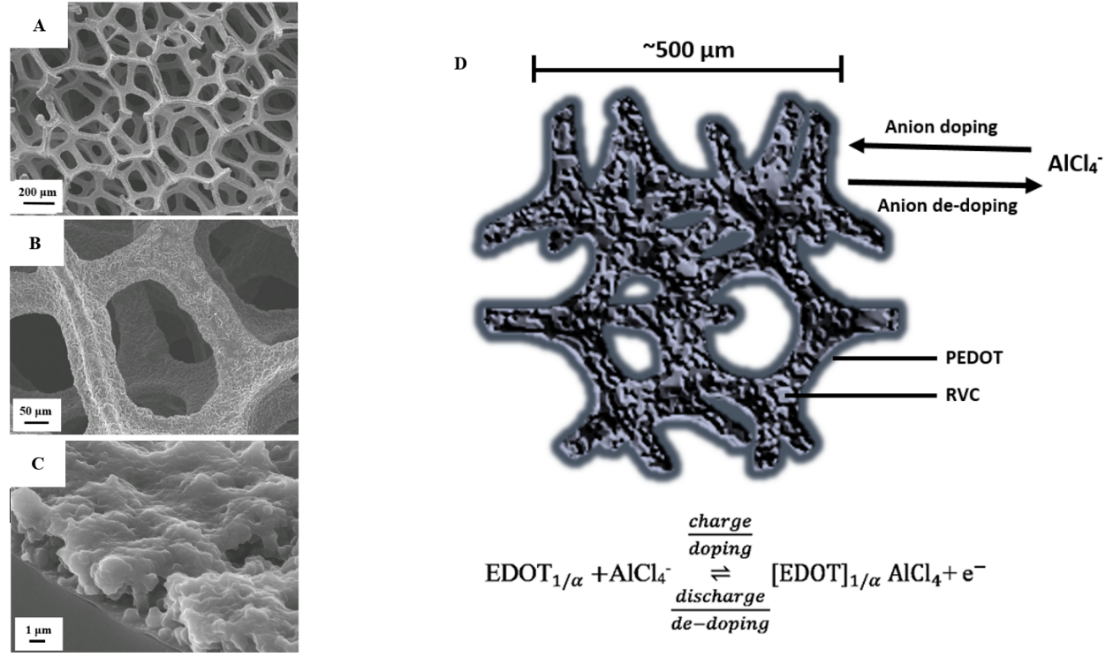 The research project included the proof-of-concept that comprises the characterisation of electrode reactions, demonstration of the battery concept and preliminary battery performance. This was followed by improvements of the electro-polymerisation of the conductive polymer films in ionic liquid and their characterisation and synthesis as 2D-micro/nano polymer films on macro-porous carbon substrates and on 3D carbon electrodes as shown on the left figure.
The research project included the proof-of-concept that comprises the characterisation of electrode reactions, demonstration of the battery concept and preliminary battery performance. This was followed by improvements of the electro-polymerisation of the conductive polymer films in ionic liquid and their characterisation and synthesis as 2D-micro/nano polymer films on macro-porous carbon substrates and on 3D carbon electrodes as shown on the left figure.
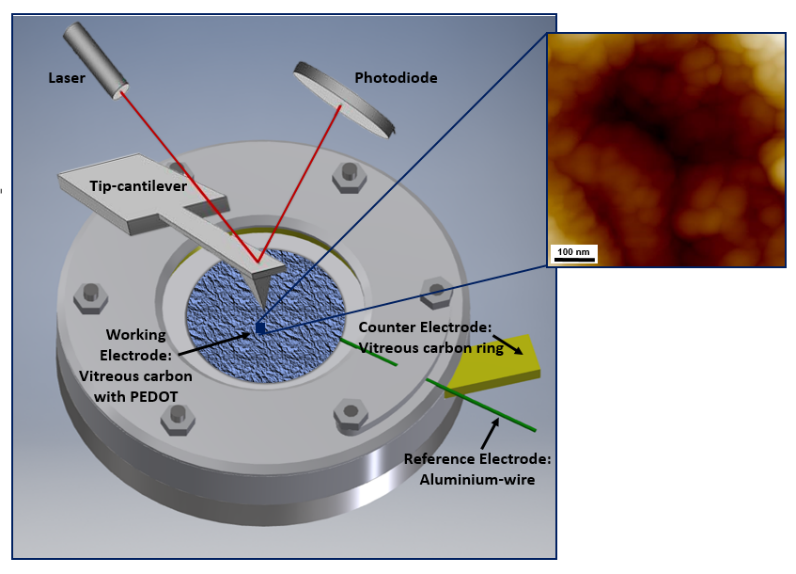
After the successful synthesis of the conductive polymer electrode, the following steps towards a complete battery system was the fundamental research of the polymer in operando. This was achieved by in-situ electrochemical quartz crystal measurements (EQCM) coupled with in-operando atomic force microscopy (AFM). This technique was useful to evaluate the doping/de-doping level, determination transferred total charges, distinction battery- or capacitor-like and visualisation of the polymer expansion and contraction on planar vitreous carbon in EMImCl-AlCl3 + EDOT, see figure below:
The battery testing and characterisation was the last part of the project and included assembling laboratory-scale battery test cells
The overall impact of the project is reflected in several publications in high impact factor journals and national and international conferences. The continuation of the project was secured by the award of a Lloyds Register Foundation PhD scholarship.
The following references authored by Dr T. Schoetz were published as a result of this PhD project:
https://doi.org/10.1007/s10008-017-3658-4
https://doi.org/10.1039/C7FD90093G
https://doi.org/10.1039/C8TA06757K
https://doi.org/10.1016/j.electacta.2018.01.033
https://doi.org/10.1016/j.elecom.2018.02.018
https://doi.org/10.1149/1945-7111/ab7573
https://doi.org/10.1016/j.est.2019.101176